Which Best Describes the Structure of an Atom
Neutrons are in motion around a nucleus that contains protons and electrons. Vastly empty space with a heavy positively charged center.

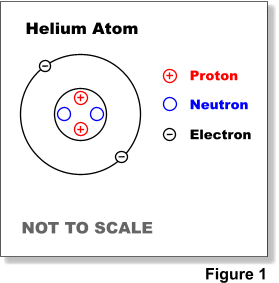
Atomic Structure Electrons Protons Neutrons And Atomic Models
The number of protons neutrons and electrons is always the same E.

. Which of these describes the structure of an atom. Examine the structure of this atom. In the experiment he used a source of alpha particles an atom and a detector.
A nucleus made up of protons surrounded by an electron cloud. His first conclusion was that most of the space inside the atom is empty. Which of the following best describes the structure of an atom.
Electrons and protons are in motion around a nucleus that contains neutrons. A nucleus made up of protons and neutrons surrounded by. Negative protons and positive neutrons in the nucleus are surrounded by neutral electrons.
A solid sphere unique for everything that exists. A tiny nucleus of protons and neutrons with electrons orbiting around it option d a large core of protons and electrons surrounded by neutrons. Which of the following best describes the structure of an atom.
An atom consists of three sub atomic particles. Around the year 1911 British physicist Ernest Rutherford carried out the famous Gold foil experiment to understand the structure of an atom. A nucleus that is mostly empty space with charged particles circling around it.
There are no comments. Protons are in motion around a nucleus that contains electrons and neutrons. This answer has been confirmed as correct and helpful.
The nucleus has a negative charge and is surrounded by positively charged electrons. An atom with which atomic diagram has chemical properties most similar to calcium. The nucleus contains negatively charged protons.
A central nucleus containing protons and neutrons with electrons orbiting in levels of high probability. Add an answer or comment. Atomic Structure Test Review.
A sphere made up of an equal number of protons neutrons and. Protons have positive charges located in the nucleus. A neutral nucleus circled by particles with positive and negative charges.
Which of these phrases best describes an atom. Neutrons have negative charges located in the nucleus. There are always the same number of protons and electrons.
The nucleus has a positive charge and es surrounded by negatively charged electrons. There are always the same number of neutrons and electrons. There are always the same number of protons and neutrons.
A positive core surrounded by electrons packet tightly around it option b. Electrons have neutral charges located in the electron cloud. Rutherfords Atomic Structure.
A central nucleus containing protons and neutrons with electrons orbiting in levels of high probability. Electrons in set orbits. Atomic Structure Test Review.
Lightweight positively charged pieces surrounding a heavy negatively charged center. A solid sphere with electrons and protons embedded. The planets represent electrons while the sun represents the nucleus.
Protons and neutrons are in the nucleus with electrons orbiting the nucleus. A positively charged nucleus circled by widely spaced negatively charged particles. - accurately describes the structure of an atom.
The current model of an atom is best described by the Solar System. Added 852019 113314 AM. Which distinguishes an atom of one element from an atom of a different element.
Group of answer choices. Which statement best describes the electronic charges and location of the subatomic particles in an atom. The mass of ethanol needed to fill the tube is found to be 4523 g.
Which correctly describes the structure of an atom. Which of the following statements best describes the basic structure of an atom. 1Which of the following best describes an atom.
The density of ethanol is 0789 gmL. Neutral protons and negative neutrons in the nucleus are surrounded by positive electrons. Which statement best describes the structure of an atom.
Which statement describes the structure of an atom. These are found in the nucleus of the atom. Positively charged subatomic particle forming part of the nucleus of an atom and determining the atomic number of an element.
All atoms of hydrogen are alike in that each atom has the same. Describe the structure of a typical atom. Calculate the inner diameter of the tube in centimeters.
A protons and electrons grouped together in a random pattern B protons and electrons grouped together in an alternating pattern C a core of protons and neutrons surrounded by electrons D a core of electrons and neutrons surrounded by protons. The nucleus contains positively charged electrons. 2 on first ring 2 on second ring.
They are protons neutrons and electrons. Positive protons and negative electrons in the nucleus are surrounded by neutral neutrons. Log in for more information.
The smallest possible amount of matter which still retains its identity as a chemical element consisting of a nucleus surrounded by electrons. Charge and mass evenly scattered throughout a spherical shape. A particle comprised of a mixture of protons electrons and neutrons option c.
A 250-cm-long cylindrical glass tube sealed at one end is filled with ethanol.

What Is The Basic Structure Of The Atom Chemistry Question
0 Response to "Which Best Describes the Structure of an Atom"
Post a Comment